Sodium Atom vs Sodium Ion
The elements in the periodic table are not stable except the noble gases. Therefore, elements try to react with other elements, to gain the noble gas electron configuration to achieve stability. Likewise, sodium also has to get an electron to achieve the electron configuration of the noble gas, Neon. All nonmetals react with sodium forming sodium ions. Except some similarities, sodium atom and sodium ion has different physical and chemical properties due to the change of one electron.
Sodium Atom
A single atom will not weigh 22.98 whatever amu. Well 1 atom of sodium is 23 amu (most likely) or 22 or it could be any other mass equal to or greater than 11 amu. 1 atomic mass unit = 1.66053886 ×. All nonmetals react with sodium forming sodium ions. Except some similarities, sodium atom and sodium ion has different physical and chemical properties due to the change of one electron. Sodium, which symbolized as Na is a group 1 element with the atomic number 11. Sodium has properties of a group 1 metal. We would like to show you a description here but the site won’t allow us. The Ariel Atom is such a product. No doors, no roof, no compromise. It’s unique, it's original and nothing else comes close.
Na Atomic Wt

Sodium, which symbolized as Na is a group 1 element with the atomic number 11. Sodium has properties of a group 1 metal. The atomic weight of it is 22.989. Its electron configuration is 1s2 2s2 2p6 3s1. Sodium is the first element in the third period, so the electrons have started to fill into orbital 3. Sodium exists as a silvery color solid. But sodium reacts very rapidly with oxygen when it is exposed to air, thus makes an oxide coating in dull color. Sodium is soft enough to cut by a knife, and as soon as it cuts, the silvery color disappears due to the oxide layer formation. Density of sodium is lower than that of water, so it floats in water while vigorously reacting. Sodium gives a brilliant yellow flame when burns in the air. Boiling point of sodium is 883 °C, and the melting point is 97.72 °C. Sodium has many isotopes. Among them, Na-23 is most abundant with the relative abundance of around 99%. Sodium is an essential element in living systems to maintain osmotic balance, for nerve impulse transmission and so on. Sodium is also used to synthesize various other chemicals, organic compounds, soap and for sodium vapor lamps.
Sodium Ion

When sodium atom releases its valence electron to another atom, it forms a monovalent (+1) cation. It has an electronic configuration of 1s2 2s2 2p6, which is similar to the electronic configuration of neon. Removal of an electron from this is hard; therefore, the ionization energy is very high (4562 kJ·mol−1). Electronegativity of sodium is very low (according to the Pauling’s scale it is about 0.93), allowing it to form cations by donating an electron to a higher electronegative atom (like halogens). Therefore, sodium often makes ionic compounds.
Na Atomic Size
What is the difference between Sodium Atom and Sodium Ion? • Sodium ion has obtained a stable electronic configuration by giving out one electron from the sodium atom. Therefore, sodium ion has one electron less than the sodium ion. • In other words, the valence shell/ last shell of sodium atom has only one electron. But in sodium ion the last shell has 8 electrons. • Sodium ion has a +1 charge whereas sodium atom is neutral. • Sodium atom is very reactive; therefore, won’t find free in nature. It exists as sodium ions in a compound. • Because of the release of one electron, the sodium ion radius differs from the atomic radius. • Sodium ion is attracted to negatively charged electrodes, but sodium atom is not. • The first ionization energy of sodium atom is very low compared to the ionization energy of sodium +1 ion. |
Related posts:
The sodium atom contains 11 electrons, 11 protons, and 12 neutrons. What is the mass number of sodium?
2 Answers
Explanation:
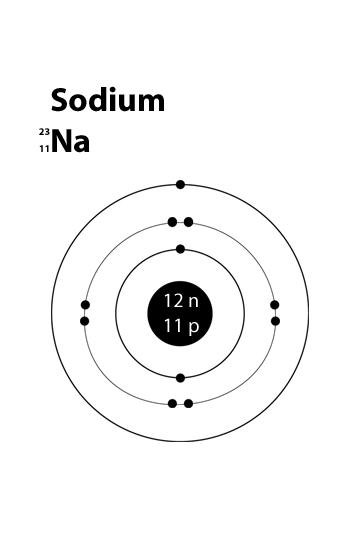
The mass number is the sum of the neutrons and protons in an atom.
So 11 protons + 12 neutrons= 23
We have the
Na Atom Drawing
Explanation:
A sneakier question would have asked:
The sum of neutrons and protons, the massive nuclear particles, gives the mass number, with which we often label the elemental symbol as a left hand superscript. But the number of
It is not too difficult to incorporate these ideas into your understanding, and if you have a Periodic Table in front of you (and you should have one NOW!) these problems are straightforward.
Na Atomic Mass Rounded
Related questions
