
The halogen atom may leave with its bonding pair of electrons to give a halide ion which is stable – a halide is called a good leaving group. If an atom replaces the halide the overall reaction is a substitution. If the halide loss is accompanied by the loss of another atom, the overall reaction is called an elimination. The halogen atom is bonded directly to the sp 2-hybridized carbon of a C=C. Aryl halides (Haloarenes) In Haloarenes, the halogen atom is bonded directly to the sp 2-hybridised carbon atom of an aromatic ring. These are also referred to as haloarenes.
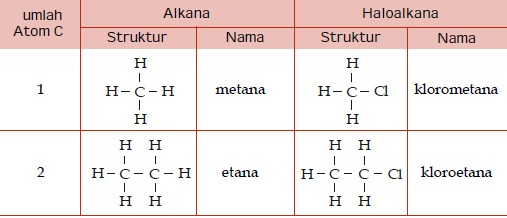
The organic compounds containing halogen atoms as part of their molecules are referred to as organohalogen compounds. Haloalkanes (alkyl halides), haloarenes (aryl halides), acid halides are some important class of these compounds.
Their molecules contain polar C-X bond as functional group; where X is a halogen atom like F, Cl, Br and I. The carbon atom carries partial positive charge and halogen atom, being more electronegative than carbon, carries partial negative charge.
The organohalogen compounds find many applications in day to day life and in industry. They are used as solvents in laboratory and in industries (like chloroform, carbon tetrachloride, westron, westrosol); as precursors to other organic molecules; as anesthetics (e.g. halothane); as refrigerants (like freons); etc.
Chloramphenicol, an antibiotic containing halogen is used for the treatment of typhoid fever. Thyroxine is an iodine containing harmone produced by thyroid glands. Polyvinylchloride, PVC is a polymer containing chlorine atoms.
Simple organohalogens like alkyl halides are obtained by replacing one or more H atoms by X atom(s) from hydrocarbons. These compounds can be classified based on hybridization of carbon to which the halogen is bonded.
Classification based on hybridization of carbon
Compounds containing sp3 C–X Bond
This class of organohalogen compounds includes:
i) Alkyl halides (or haloalkanes)
ii) Allylic halides
iii) Benzylic halides

Alkyl halides (or haloalkanes)
Haloalkanes are represented by R–X. The X atom is bonded to sp3 hybridized carbon of an alkyl group, R that is derived from an alkane.
Monohaloalkanes are represented by general formula CnH2n+1X. These are obtained by replacing one of the hydrogen from alkane by halogen.
| Alkane | minus H plus X = | Mono haloalkane |
| CnH2n+2 | - H + X = | CnH2n+1X |
For example, methyl chloride, CH3-Cl is obtained by replacing one hydrogen atom of methane by chlorine.
CH4 - H + Cl = CH3-Cl
They are further classified into primary (1o), secondary (2o) and tertiary halides (3o) according to the nature of carbon to which halogen is bonded.
E.g.
Primary: ethyl chloride - the chlorine atom is attached to primary carbon.
Secondary: isopropyl chloride - the chloro group is attached to a secondary carbon.
tertiary: tert-butyl chloride - the chlorine atom is bonded to a tertiary carbon.
Allylic halides
In allylic halides, the halogen is bonded to an sp3–hybridized carbon atom that is adjacent to a C=C. This carbon is also know as allylic carbon.
E.g. Allyl chloride.
They show special trend in the reactivity due to stability of allyl carbocation.
Benzylic halides
In benzylic halides, the halogen group is bonded to an sp3-hybridized benzylic carbon atom that is inturn bonded to an aromatic ring.
E.g. Benzyl bromide.
These are also more reactive than normal haloalkanes due to stability of benzyl carbocations.
Compounds containing sp2 C–X bond

This class includes :
i) Vinylic halides
ii) Aryl halides
iii) Acid halides
Vinylic halides
Halogen Atomic Mass
The halogen atom is bonded directly to the sp2-hybridized carbon of a C=C.
E.g. vinyl fluoride.
Aryl halides (Haloarenes)
In Haloarenes, the halogen atom is bonded directly to the sp2-hybridised carbon atom of an aromatic ring. These are also referred to as haloarenes.
E.g. Chlorobenzene
Both vinylic and aryl halides are less reactive towards nucleophilic substitution reactions due to stronger C-X bond.
Acid halides
Acid halides are the derivatives of carboxylic acids. These are obtained by substituting -OH group of carboxylic acid by any halogen atom. In this case, the X atom is attached to an sp2 hybridized carbonyl carbon.
The chemistry of these compounds is discussed along with other acid derivatives separately.
Polyhalogenated compounds
The organohalogen molecules may contain one or more halogens also. They are referred to as dihalo, trihalo, tetrahalo, poly halo compounds.
Dihalo substituted compounds:
E.g. Methylene chloride, CH2Cl2
If two halogen groups are present on one carbon, those are termed as geminal dihalides (e.g. Ethylidene dichloride) and if two halogen atoms are present on adjacent carbon atoms, then those are referred to as vicinal dihalides (e.g. Ethylene dichloride).
Halogen Atom Transfer
Trihalo substituted:
E.g.
Chloroform, CHCl3,
Westrosol (or Trichloro ethylene), CHCl=CCl2
Tetrahalo substituted:
E.g.
Carbontetrachloride, CCl4
Westron (or Acetylene tetrachloride), CHCl2-CHCl2
Perhalo compounds: When all the H atoms are substituted by X atoms, those compounds are generally referred to as perhalo compounds.
E.g. Perchlorodiethyl ether
| Organic chemistry: Home page | Nomenclature of organohalogen compounds > |
